- Title
-
The zebrafish forkhead transcription factor Foxi1 specifies epibranchial placode-derived sensory neurons
- Authors
- Lee, S.A., Shen, E.L., Fiser, A., Sali, A., and Guo, S.
- Source
- Full text @ Development
|
The no soul embryos are defective in visceral sensory neurons. Anterior is to the left. Lateral views of wild-type (left) and no soul (right) embryos expressing islet-GFP (A-J) or labeled with the monoclonal antibody mAb16A11 (K-L). (A-D) 26 hpf embryos showing that the VIIth ganglia are not generated in the mutant, whereas cranial motor neurons appear normal. (E-H) 48 hpf embryos showing that the VIIth and IXth ganglia are absent while the Xth ganglia are reduced. (I,J) ~3-day old embryos showing persistent defects in visceral sensory neurons of the mutant. (K,L) Anti-Hu antibody labeling of 50 hpf embryos showing that the trigeminal ganglia appear normal, while both epibranchial and lateral line ganglia are defective in the no soul mutant. The identity of the no soul mutant embryos shown in all figures is confirmed by genotyping with closely linked polymorphic markers Z1400 and Z13938. AD, anterior dorsal ganglia; AV, anterior ventral ganglia; M, middle lateral line; mV/mVII/mX, the Vth, VIIth, or Xth motor neurons; ov, otic vesicle; OT, ocular and trochlear motor nuclei; P, posterior lateral line; V, trigeminal ganglion; VII, geniculate ganglion; IX, petrosal ganglion; X, nodose ganglion. Scale bar: 100 μm (A-B, E-F, K-L), 70 μm (C-D, G-J). EXPRESSION / LABELING:
PHENOTYPE:
|
|
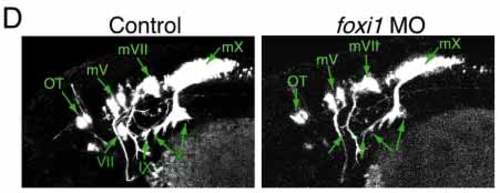
The no soul gene encodes a winged helix domain-containing protein that belongs to the foxi1 subfamily. (A) ABI automated sequencer-produced chromatographs showing that the Ser194 is mutated to Pro in the no soul mutant. (B) Comparative model showing that the predicted conformations of the native and mutated segment are different. The ab initio modeled segment is in green (the rest of the protein is shown in brown), the proline mutation is shown in the ball-and-stick style (red), and the bound DNA is blue. In the mutant model, the C terminus of the recognition helix is shortened by one turn and the loop connecting the recognition helix to the subsequent b strand is shifted significantly relative to the native model. These conformational changes decrease the extent of interactions between the protein and DNA, assuming DNA is positioned as in the template structures. (C) Sequence alignment between no soul/foxi1, Xenopus (xfoxi1c), mouse (Mfkh10) and the winged helix domain of genesis that was used for comparative structure modeling. The winged helix motif and the position of the no soul mutation are marked. (D) Confocal image showing the control and the foxi1 morpholino-injected embryos. Although motor neurons are normal, epibranchial placode-derived visceral sensory neurons are defective as in the no soul mutant. mV/mVII/mX, the Vth, VIIth and Xth motor neurons; OT, ocular and trochlear motor nuclei; VII, geniculate ganglion; IX, petrosal ganglion; X, nodose ganglion. |
|
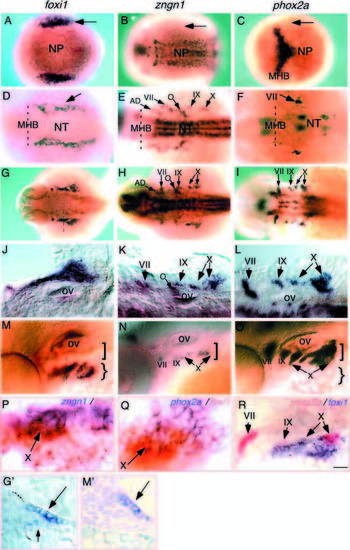
Spatial and temporal expression of no soul/foxi1, ngn and phox2a. In situ hybridization with antisense no soul/foxi, ngn, or phox2a RNA probe. Anterior is to the left, except in G and 3M inset, where dorsal is at the top. (A-C) Dorsal view of tailbud stage embryo showing no soul/foxi1 expression in the lateral cranial placodal domain (epibranchial and otic placodes; arrows), while ngn and phox2a are not yet detectable in this domain. (D-F) Dorsal views of 24 hpf embryos showing persistent expression of foxi1 (arrow) in the lateral cranial placodal region, while ngn and phox2a expression are detected in the vicinity of foxi1. (G-L) Dorsal views of 36 hpf embryos showing that all three genes are expressed in close proximity to one another. (G′; below) A cross section through the foxi1-expressing domain: the large arrow points to foxi1-expressing cells in the ectodermal layer, while the small arrow points to cells weakly expressing foxi1 that are likely delaminating neural precursors. (M-O) Lateral views of 48 hpf embryos. (M′; below) A cross section through the foxi1-expressing domain: the arrow points to the foxi1-expressing cells. (P,Q) Double in situ hybridization showing the overlapping expression of foxi1 (red) and ngn (P) or phox2a (Q) (purple). The arrow indicates the nodose (X) ganglionic region. (R) Double in situ hybridization showing the overlapping expression of phox2a (red) and foxi1 (purple). All three epibranchial placode-derived sensory ganglionic regions are indicated with arrows. AD, anterior dorsal lateral line; MHB, mid-hindbrain boundary; NP, neural plate; NT, neural tube; O, octaval/statoacoustic ganglion; ov, otic vesicle; VII, geniculate ganglion; IX, petrosal ganglion; X, nodose ganglion. Scale bar: 150 μm (A-F, G-I), 100 μm (M-O), 50 μm (J-L,R), 25 μm (G′,M′), 10 μm (P,Q). |
|
Foxi1-expressing progenitor cells are present at 24 hpf but fail to initiate ngn and phox2a expression in the no soul mutant. Anterior is to the left in A-L, and dorsal is to the top in M-N. (A-D) 24 hpf embryos showing that the foxi1-expressing domain is still intact in the no soul mutant and is apparently upregulated. (E-H) 24 hpf embryos showing that ngn and phox2a expression in the epibranchial placodes fail to be initiated in the no soul mutant. (I-L) Double in situ showing that while foxi1-expressing cells (red) are still present, they do not express ngn or phox2a (purple) in the no soul mutant. (M-P) Cross sectioning through the foxi1 (purple)- and phox2a (red)-expressing domain in 24 hpf embryos, showing that foxi1-expressing cells do not appear to delaminate and no phox2a+ sensory neurons are generated in the no soul mutant. (M,N) Nomarski views, (O,P) rhodamine fluorescence. The arrows point to the foxi1-expressing cells in the ectodermal layer, while the arrowhead points to cells that weakly express foxi1, are likely to be delaminating neural precursors, and overlapping phox2a+ epibranchial sensory neurons. AD, anterior dorsal lateral line; MHB, mid-hindbrain boundary; NP, neural plate; NT, neural tube; O, octaval/statoacoustic ganglion; ov, otic vesicle; OT, ocular and trochlear motor nuclei; VII, geniculate ganglion; IX, petrosal ganglion; X, nodose ganglion. Scale bar: 100 μm (A-B, E-H), 50 μm (C-D, I-L), 40 μm (M-P) 25 &mum. |
|
no soul/foxi1-expressing cells later disappear in the no soul mutant. Anterior is to the left. (A-B) 36 hpf embryos labeled with foxi1 showing that the anteriorly located foxi1-expressing cells are absent in the no soul mutant. (C-F) 36 hpf embryos labeled with ngn (C,D) and phox2a (E-F) showing their expression in the visceral sensory neurons are largely absent. (G,H) 36 hpf embryos labeled with phox2b showing that its expression in the VIIth and IXth ganglia is absent, while its expression in the Xth ganglia appear normal in the mutant. (I,J) 60 hpf embryos labeled with phox2a showing that the deficits continue in the no soul mutant. AV, anterior ventral lateral line; MHB, mid-hindbrain boundary; O, octaval/statoacoustic ganglion; OT, oculomotor and troclear motor nuclei; VII, geniculate ganglion; IX, petrosal ganglion; X, nodose ganglion. Scale bar: 100 μm. |
|
TUNEL staining of whole-mount wild-type (left) and no soul mutant (right) embryos. C,D,G,H,K,L are higher magnification views of A,B,E,F,I,J, respectively. (A-D) 24 hpf embryos showing comparable TUNEL staining in wild-type and no soul mutant embryos. (E-H) 28 hpf embryos showing increased TUNEL staining in the anterior lateral cranial placodal region. (I-L) 36 hpf embryos showing increased TUNEL staining in the posterior cranial placodal region. MHB, mid-hindbrain boundary; ov, otic vesicle. Scale bar: 100 μm (A,B,E,F,I,J), 50 μm (C,D,G,H,K,L). |
|
Ectopic expression of foxi1. Anterior is to the left. (A,B) Dorsal views of control embryos labeled with phox2a and ngn antisense RNA probes, respectively. (C-F) 24 hpf foxi1-injected embryos, labeled with (C) phox2a, showing ectopic phox2a+ cells on the yolk surface (arrows); (D) ngn, showing ectopic ngn+ cells on the yolk surface (arrow); (E) foxi1 (red) and phox2a (purple), showing that phox2a+ cells do express ectopic foxi1; (F) foxi1 (red) and ngn (purple), showing that ngn+ cells do express ectopic foxi1. Scale bar, 100 μm. |