- Title
-
A homeobox gene, pnx, is involved in the formation of posterior neurons in zebrafish
- Authors
- Bae, Y.K., Shimizu, T., Yabe, T., Kim, C.H., Hirata, T., Nojima, H., Muraoka, O., Hirano, T., and Hibi, M.
- Source
- Full text @ Development
|
Expression of pnx. Expression of pnx was detected by whole-mount in situ hybridization. (A,B) 50% epiboly stage. Lateral view (A) and animal pole view (B). Dorsal is towards the right. pnx was expressed in the entire ectoderm exclusive of the marginal blastoderm (arrowhead in A). (C-F) 70%-epiboly stage. Dorsal view (C), lateral view (D), animal pole view (E) and sagittal section of the dorsal marginal region (F). (D-F) Dorsal is towards the right. The pnx transcripts were detected in the ventrolateral and dorsomedial regions of the ectoderm at the mid-gastrula stage. (G,H) 85% epiboly stage. Dorsal view (G) and lateral view (H). (I-K) One-somite stage. (I) Dorsal view. (J) Two-color staining with pnx (purple) and krox20 (red). (K) Horizontal section of the pnx-stained tail-bud state embryos (m, primary motoneurons; i, primary inter neurons; r, Rohon-Beard neurons). From the late gastrula to early segmentation stages, the pnx transcripts became restricted to three stripes of primary neurons posterior to rhombomere 4 (the krox20 expression indicates rhombomeres 3 and 5). (L) Ten-somite stage (14 hpf), lateral view. (M) Prim-6 stage (25 hpf), lateral view. The expression of pnx gradually disappeared from the anterior region from the late segmentation stages and it was only detected in the caudal neural tube at the early pharyngula stage. EXPRESSION / LABELING:
|
|
Regulation of pnx expression. (A-C2) Expression of pnx in wild-type (A,A2), chordino (B,B2) and swirl (C,C2) mutant embryos at the mid-gastrula stage (85% epiboly). (A-C) Dorsal views. (A2-C2) Vegetal pole views, with dorsal towards the bottom. Arrows and arrowheads indicate the ventral and dorsal limit of lateral expression domains of pnx, respectively. Lateral expression domains of pnx, which correspond to the primary inter neurons and Rohon-Beard (RB) neurons, became closer to the dorsal midline in chordino mutant embryos. By contrast, the pnx transcripts were detected in the entire ectoderm margin at the mid gastrula stage in swirl mutant embryos. (D-I) Reciprocal regulation of pnx by prospective posteriorizing and anteriorizing signals. The expression of pnx was expanded or ectopically induced at the three-somite stage in the embryos injected with 0.5 pg of sqt RNA (E) and 10 pg of fgf8/ace RNA (F), or treated with 1 μM retinoic acid at the gastrula stage (G) (D, control). (D-F) Dorsal views and (G) lateral view. (H,I) pnx expression was reduced and the expression domain was posteriorly shifted in embryos injected with 20 pg of dkk1 RNA (H) and 1 pg of antivin (I) RNA at the three-somite stage. Dorsal views. (J-L2) Non-axial mesendoderm induced the ectopic expression of pnx and hoxb1b in the forebrain region. (J) Procedure of the experiment. Marginal blastomere cells from the rhodamine-dextran-injected embryos were transplanted into the animal-pole region of host embryos at the shield stage, which were then stained at the three-somite-stage for pnx (K,K2) or hoxb1b (L,L2). Lateral views, with dorsal towards the right (K-L2). (K2,L2) Epifluorescence images of K,L, respectively. Ectopic expression of pnx and hoxb1b is indicated by arrowheads. (M-Q) Regulation of pnx by the Delta-Notch signal. The number of pnx-expressing cells at the three-somite stage in mind bomb (mib) mutant embryos (N) and embryos injected with RNA of 100 pg of XDlstu RNA (P) was increased in comparison with the wild-type non-injected control embryos (M). By contrast, the pnx-expressing cells were absent or severely reduced in number in the embryos injected with RNA of zebrafish notch5ICD (50 pg, O) or neurogenin1 (50 pg, Q). Dorsal views. EXPRESSION / LABELING:
|
|
Repressor activity of Pnx was required for the formation of posterior neurons. Schematic representation of Pnx constructs. Ten picograms of pnx, 5 pg of EnRpnx or 20 pg of VP16pnx RNA were co-injected with 50 pg of β-galactosidase RNA into one blastomere of two- to four-cell stage zebrafish embryos. The embryos were fixed at the three-somite stage, and the expression of β-galactosidase was stained by X-gal. (B-E) Overexpression of pnx in posterior neuroectoderm increased the numbers of cells expressing ngn1 (B) and elavl3 (C), whereas the overexpression of pnx in anterior neuroectoderm inhibited their expression (indicated by an arrowhead in B). Dorsal (B,C) and dorsolateral (D,E) views. (D,E) ngn1-expressing cells were increased in the right side (injected side, E) of the embryos, in which pnx and β-galactosidase RNA were localized, compared with the control side (left side, D). Expression of EnR-Pnx also elicited an increased expression of ngn1 (right side, F) and elavl3 (G). By contrast, expression of VP16-Pnx strongly inhibited the expression of ngn1 (left side, H) and elavl3 (right side, I). Highly magnified views of the pnx RNA (J,K) and VP16pnx RNA (L,M). The borderlines between the injected (lower) and the non-injected (upper) sides are indicated by broken lines. (N,O) Overexpression of pnx slightly expanded neuroectoderm expressing sox19 (N), but expression of VP16pnx did not significantly affect the formation of neural plate (O). The left sides are injected sides. |
|
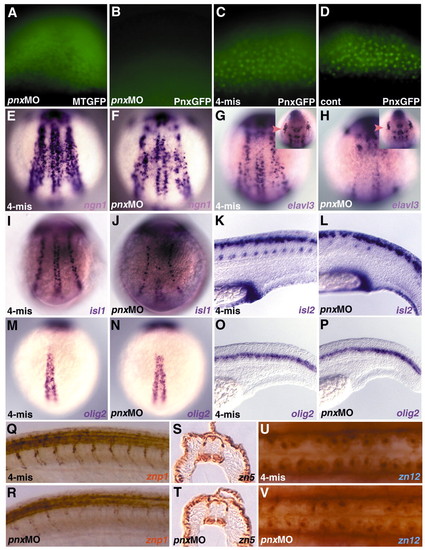
Pnx is involved in primary neurogenesis. (A-D) Specific inhibition of the translation of pnx RNA by antisense morpholino oligonucleotide (MO). Two nanograms of the MO recognizing the 5′ untranslated region of pnx (pnx MO; A,B) or the control MO (4-mis; C), which contains four mispaired bases, were coinjected with RNA for Pnx-GFP (B,C) or Myc-tagged GFP (MTGFP; A) into one-cell-stage embryos. (D) Pnx-GFP-expressing control embryo. The expression of Pnx-GFP and MTGFP was detected by fluorescence microscopy. pnx MO (F,H,J,L,N,P,R,T,V) and 4-mis MO (E,G,I,K,M,O,Q,S,U)-injected embryos were stained with markers for neural precursors and primary and secondary neurons. (F,H) ngn1- and elavl3-expressing cells were diminished in the posterior neuroectoderm of pnx MO-injected embryos at the one- to three-somite stage. (G,H) elavl3 expression in trigeminal ganglions was not significantly affected in the pnx MO-injected embryos (marked by arrowheads in insets). (I,J) At the five-somite stage, pnx MO-injected embryos displayed a strong reduction in islet1-expressing primary motoneurons and a less efficient reduction in islet1-positive Rohon-Beard neurons. (K,L) At 24 hpf, the pnx MO-injected embryos (L) displayed a reduction in islet2-expressing primary motoneurons and defects in axon outgrowth from motoneurons (stained with the znp1 monoclonal antibody, R). (M-P) olig2 expression was not affected in the pnx MO-injected embryos at the one-somite stage (N) and at 24 hpf (P). (S,T) zn5-immunoreactive secondary motoneurons (48 hpf) and (U,V) zn 12-immunoreactive Rohon-Beard neurons (25 hpf) appeared to be normal or only marginally affected in the pnx MO-injected embryos (T,V). (E-J,M,N) Dorsal views. (K,L,O-R) Lateral views. (S,T) Horizontal sections in the spinal cord region. (U,V) Dorsal views in the spinal cord region. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Pnx can promote primary neurogenesis independently of Delta-Notch signaling. (A-H) Ten picograms of pnx and β-galactosidase RNA, 20 pg of VP16pnx and β-galactosidase RNA, or 2 ng of pnx MO (recognizing the translational initiation site) were injected into embryos of crossed mib mutants, fixed at the three-somite stage, and stained with ngn1, elavl3 and islet2 (A-H). The blue region stained with X-gal contained the injected RNA (A-D). The mib homozygous mutant embryos were detected by an increase in Rohon-Beard neurons. Misexpression of pnx in mib expanded the ngn1- (A) and elavl3-expressing stripes (B) but did not change the density of those cells within the stripes. Expression of VP16-Pnx (C,D) or injection of pnx MO (E,F) into the mib mutant embryos reduced the numbers of ngn1 (C,E,F) and elavl3-expressing cells (D) at the three-somite stage. islet2-expressing primary motoneurons were reduced in the pnx MO-injected mib mutant embryos (H). (I-N) One hundred picograms of XDlstu RNA and β-galactosidase RNA were co-injected with 10 pg of pnx RNA or 20 pg VP16pnx RNA into the wild-type embryos, and the expression of ngn1 and elavl3 at the three-somite stage was detected. Expression of XDlstu increased the density of ngn1- (I) and elavl3-expressing cells (J), and co-expression of Pnx expanded the ngn1- (K) and elavl3-expressing (L) stripes without affecting the densities of ngn1- and elavl3-positive cells. Co-expression of VP16-Pnx reduced the numbers of ngn1- (M) and elavl3-expressing cells (N). (A-F,I-N) Dorsal views. (G,H) Lateral views in the spinal cord region. (O) Model for the role of pnx in posterior neurogenesis. EXPRESSION / LABELING:
PHENOTYPE:
|