- Title
-
Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis
- Authors
- Henry, C.A., Crawford, B.D., Yan, Y.-L., Postlethwait, J., Cooper, M.S., and Hille, M.B.
- Source
- Full text @ Dev. Biol.
|
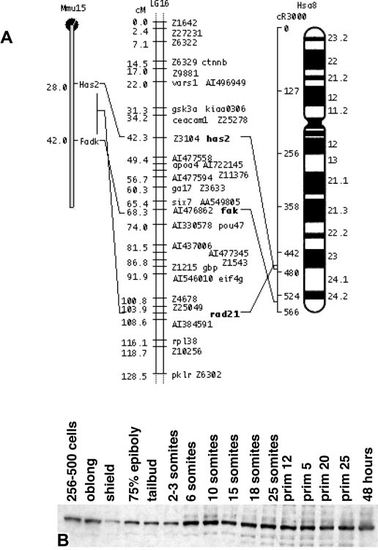
Conserved syntenies support the conclusion that fak and PTK2 are orthologues. Three loci on LG 16 have apparent orthologues on human (Hsa) chromosome 8q and mouse (Mmu) chromosome 15. These three loci are (in order, fish/human/ mouse): fak,/PTK2/Fadk; rad21(AI477810)/RAD21/Rad21; has2(U53223)/HAS2/Has2. Other named loci in the figure are apparent orthologues of loci on other human chromosomes (Woods et al., 2000). It is interesting to note that while PTK2/FAK/FADK is at Has 8q24-qter, the closely related gene PTK2B/CAKβ is distantly located on the same chromosome at Has 8p21. (B) Fak protein is expressed throughout early development and expression increases slightly during somitogenesis. Ten embryos were added per lane. |
|
fak mRNA is expressed in the notochord, somite, and unsegmented paraxial mesoderm in wild-type zebrafish embryos. (A–F, H) Dorsal view. (G) Transverse view. “S” indicates somite stages. (A, B) Expression of fak in the notochord (red arrows) and somites (black arrows) in early embryos. (C) The black arrow points to the posterior border of the eighth (last formed) somite. (D) Expression of fak in somites (black arrow) in a late embryo. The absence of notochordal fak is indicated at the red arrow. (E, H) Two-color in situ hybridization (H is enlarged view of the box region in E) of the localization of fak (blue) and the two strong bands of papc expression (red) in the unsegmented paraxial mesoderm. (F) fak is not expressed in the adaxial cells that abut the notochord (black arrow). (G) A cross-section of an 18-somite embryo indicates that fak is expressed throughout the region of the notochord that is still undergoing cell intercalation (red arrow) and throughout the dorsal–ventral extent of the somites (black arrow). EXPRESSION / LABELING:
|
|
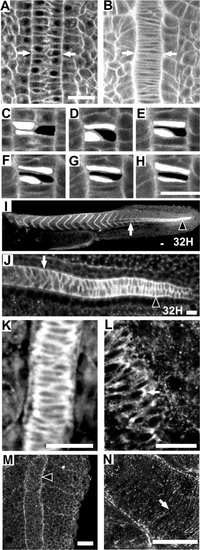
Zebrafish notochord cells intercalate as in Xenopus embryos; Fak protein is highly expressed in the region of the notochord that is undergoing convergence; and Fak is phosphorylated at the notochord-somite boundary. Anterior is towards the top or left in all panels. Dorsal views except (I) and (J), which are side views. (A–H) Time-lapse analysis of a vitally-stained zebrafish embryo (see methods) from the 1-somite stage through the 4-somite stage at the level of the first 4 somites. At time 0 min (A), the notochord is two cells wide (white arrows) and by time 38 min (B) cellular intercalation results in a notochord that is one cell wide. (C–H) Enlarged view of three cells intercalating. (C) 0 min. (D) 10 min. (E) 23 min. (F) 30 min. (G) 35 min. (H) 40 min. (I–L) Immunostaining for Fak protein. The posterior region of the notochord undergoing cell intercalation is noted with an arrowhead. White arrow indicates the notochord–somite boundary anterior to the intercalating domain. (K) A 10-somite embryo, dorsal view at the level of somite 4 showing the notochord–somite boundary. (L) Enlarged view of a notochord undergoing intercalation in a 10-somite embryo, at the level of somite 8. An arrow notes a discrete plaque of Fak. (M, N) Immunostaining for pY397FAK. M: A 13-somite embryo at somite 13 stained for phospho-Fak at the notochord-somite boundary (black arrowhead). (N) Projection of confocal sections that include the entire thickness of notochord of a 13-somite embryo showing circumferential striations in phospho-Fak staining perpendicular to the notochord axis. Scale bars, 20 μm, with the exception of (I), where scale bar is 40 μm. |
|
Fak protein and phosphorylated Fak become concentrated at somite boundaries during somitogenesis and persist throughout myotome development. (A–D, G) Dorsal views. (E, F, H) Side views. (A–F) Fak stained with anti-human Fak. (G, H) Phospho-Fak stained with anti-pY397FAK. (A) 10-somite embryo (white arrow designates last formed somite). (B) 7-somite embryo. White arrow designates a somite boundary. (C) A higher magnification of Fak in a 10-somite embryo. Arrow indicates the basal side of border cells that abut the intersomitic furrow (arrow). (D) 7-somite embryo. Arrow indicates the lateral edges of the lateral epithelial cells of a somite. (E, F) 32-h embryos. (G) 10-somite embryo. (H) 30-h embryo. Scale bars, 20 μm, with the exception of (E), where scale bar is 40 μm. |
|
Fak protein localizes to somite boundaries in kny;tri double-mutant embryos that have somites comprised of only two rows of epithelial border cells. (A–C) In situs for fak mRNA expression in 9-somite embryos (dorsal views with anterior up). (A) Wild-type embryo. (B) knypek or trilobite embryo. (C) knypek; trilobite embryos. (D) Immunostaining for Fak protein in a 10- somite kny;tri embryo (dorsal view with anterior left). Scale bars, 20 μm. |
|
Notch signaling through Suppressor of Hairless is not necessary for normal fak mRNA expression; but, ectopic Suppressor of Hairless signaling disrupts the normal segmental pattern of fak mRNA expression. Black arrows indicate the side of the embryo expressing the construct, while white arrows indicate the control side. (A, C) Embryos viewed with Hoffman optics. (B, D) In situ hybridization for fak mRNA on the same embryo viewed in bright field. (A, B) A live 10-somite embryo that was injected with a dominant-negative form of Suppressor of Hairless, X-Su(H)1DBM at the two- to four-cell stage. (C, D) A live 10-somite embryo that was injected with an activated form of Suppressor of Hairless, X-Su(H)1/Ank at the two- to four-cell stage. Scale bars, 20 μm. |
|
fak mRNA expression is disrupted in the fss-type mutants. The percentages denote the percentage of mutant embryos that showed a particular type of fak expression. Red arrows denote the last formed somite, black arrowheads denote ectopic fak expression in adaxial cells, and black arrows designate nonsegmental fak expression. (A, D, G, J) Wild-type embryos at the 10-, 11-, 9-, and 10-somite stages, respectively. All mutant embryos were cultured with their wild-type siblings. (B, C) fused somite (fss) embryos, which do not form somites. (E, F) beamter (bea) embryos which form one to four somites. (H, I) deadly seven (des) embryos which form 5- to 9-somites. (K, L) after eight/DeltaD (ei/DeltaD) embryos which form 5- to 9-somites. Scale bar in (J), 20 μm. |
Reprinted from Developmental Biology, 240(2), Henry, C.A., Crawford, B.D., Yan, Y.-L., Postlethwait, J., Cooper, M.S., and Hille, M.B., Roles for zebrafish focal adhesion kinase in notochord and somite morphogenesis, 474-487, Copyright (2001) with permission from Elsevier. Full text @ Dev. Biol.