- Title
-
Zebrafish colourless encodes sox10 and specifies non-ectomesenchymal neural crest fates
- Authors
- Dutton, K.A., Pauliny, A., Lopes, S.S., Elworthy, S., Carney, T.J., Rauch, J., Geisler, R., Haffter, P., and Kelsh, R.N.
- Source
- Full text @ Development
|
cls phenotype is rescued by ectopic sox10 expression. Wild-type embryos show many large, strongly pigmented melanophores at 48 hpf (A; close-up of anal region in D), while hs>sox10(L142Q)-injected clsm618 mutants (B) show only tiny melanised spots in position of premigratory NCCs (arrowheads). (C,E,F) By contrast, clsm618 embryos injected with hs>sox10 and heat-shocked, show mosaic rescue of melanophores. This embryo showed one rescued melanophore in the dorsal stripe (* in C), two in the ventral stripe (arrows in C; close-up in E) and one on the yolk sac (F). Scale bar: 125 μm in A-C; 70 μm in D-F. |
|
Embryonic sox10 expression in wild-type and cls embryos. (A,B) sox10 is expressed in most cranial (A; five-somite stage) and trunk (B; 14-somite stage) premigratory NCCs; double mRNA in situ hybridisation with fkd6 (O, purple) and sox10 (P, green) in a six-somite stage embryo reveals extensive overlap, although some individual NCCs lack sox10 (arrowheads). (C) 18-somite stage embryo shows strong expression in premigratory (black arrow) and migrating (asterisk) NCCs and otic vesicle (o). (D,E) Double mRNA in situ hybridisation with dlx2 (D, purple) and sox10 (E, green) in 29 hpf stage embryos shows absence of sox10 expression in developing branchial arches (1-5). (F) By 24 hpf in wild types, strong expression is associated with cranial ganglia (white arrowheads) and posterior lateral line nerve (black arrowhead; enlarged in inset), otic vesicle (o), migrating NCCs throughout trunk (asterisks) and in premigratory crest (arrow). (G) At 24 hpf in cls mutants, expression in the head is clustered (white asterisks) and cranial ganglia have reduced labelling (white arrowheads). Cells expressing sox10 extend along the posterior lateral line nerve (arrowhead and inset). Rostral trunk shows some migrating cells (black asterisk), but sox10-positive cells are clustered dorsal to the neural tube (arrows) in trunk and tail. (H,I) Combined sox10 in situ hybridisation (purple) and anti-Hu antibody labelling (orange) shows strong sox10 expression associated with wild-type (H) posterior lateral line ganglion (g), much reduced in cls mutant (I). (J) In transverse section of wild-type ganglion (approximate position indicated by white line in H), sox10 expression is strong peripherally (non-neuronal cells), but absent centrally (neurones). (K,L) 36 hpf wild-type embryos show weak expression in some melanophores, but not all. Thus, weak expression (arrowhead) is seen in some cells of the dorsal stripe (K), but not in cells on the yolk sac (L). (M,N) Number of sox10-expressing NCCs in different locations (premigratory, pre; migrating on medial pathway, MP; migrating on lateral pathway, LP) of trunk and anterior tail (somites 1-20) at 24 (M) and 30 hpf (N) in WT and cls mutants. (Q,R) Segmentally arranged lines of sox10-positive cells lying adjacent to the notochord (no), presumably glia, are abundant in wild type (Q) and only weakly affected in cls mutants (R) at 40 hpf. (S,T) Transverse section of trunk of 60 hpf wild-type embryo (S) shows enteric nervous system expression (arrowheads) lateral to the gut (g), absent in cls mutant (T). e, eye; m, muscle; s, somite; vs, ventral stripe melanophores. All images are lateral views, rostral towards the left, dorsal upwards, except dorsal views of A,K,O,P. Scale bar: 200 μm in A; 50 μm in B,H-I,K-L; 120 μm in C; 75 μm in D,E; 150 μm in F,G; 35 μm in J; 65 μm in O,P; 55 μm in Q,R; 45 μm in S,T. EXPRESSION / LABELING:
|
|
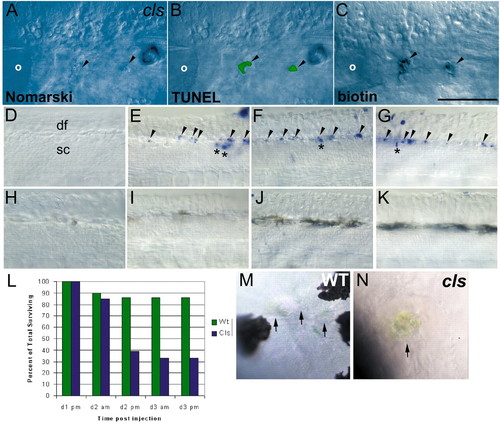
NCC death in cls embryos. (A-C) NCC clones die by an apoptotic mechanism in cls embryos. Lateral views of 40 hpf cls embryo in which two daughters of a single labelled NCC contributed to the posterior lateral line ganglion, lying just posterior to the otic vesicle (o). In the live embryo, both cells show blebbed morphology typical of apoptotic cells when viewed with Nomarski optics (A). After fixation and processing for TUNEL (B) and detection of the biotinylated-dextran lineage-tracer (C), visible TUNEL signal of these clonal cells indicates DNA fragmentation characteristic of apoptotic cells. (D-K) Whole-mount TUNEL shows NCC apoptosis in cls embryos. Lateral views of dorsal spinal cord (sc) in tail of 30 (D,H), 35 (E,I), 40 (F,J) and 45 (G,K) hpf embryos show apoptotic NCCs immediately dorsal to the spinal cord from 35 hpf in cls (arrowheads, D-G), but not wild-type (H-K), embryos. Scattered TUNEL-positive cells are prominent in dorsal spinal cord (*) of cls embryos (E-G); these are occasionally seen in wild-type siblings at these stages (data not shown). df, dorsal fin. (L) Time-course of labelled single cranial NCC clone survival in cls mutants and their wild-type siblings. Percentage of surviving clones is given at each of the five standard time points when embryos were examined. The time points correspond to approximately 16, 32, 40, 56 and 64 hpf, respectively. The first time point includes only single labelled NCCs based on examination within a few hours after labelling. See text for further details. (M,N) Wild-type xanthophores (arrows) at 48 hpf have a very flattened, thin morphology and are only weakly coloured (M), while a dying cls xanthophore (arrow) shows characteristic apoptotic morphology and concentrated yellow coloration, which was usually visible by 35 hpf (N). Scale bar: 100 μm in A-C; 50 μm in D-K; 75 μm in M,N. |
|
cls mutants lack nac and spa expression. Lateral views of caudal trunk of 25 hpf wild-type and cls embryos after in situ hybridisation with nac (A,B), spa (E,F) and dct (G,H) probes. (C,D) nac/mitf expression is decreased weakly (C) or strongly (D) after injection with either a low or high dose, respectively, of sox10 morpholino. n, neural tube; y, yolk sac. Scale bar: 75 μm. EXPRESSION / LABELING:
|