- Title
-
sucker encodes a zebrafish Endothelin-1 required for ventral pharyngeal arch development
- Authors
- Miller, C.T., Schilling, T.F., Lee, K., Parker, J., and Kimmel, C.B.
- Source
- Full text @ Development
|
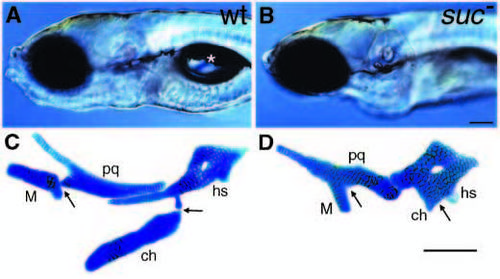
suc mutants have reduced ventral cartilages. (A,B) Lateral views of live wild-type (A) and sucker mutant (B) larvae at 4 days. Mutants lack lower jaws and do not form swim bladders (asterisk in A). (C,D) Alcian-stained cartilages from a wild type (C) and suc mutant (D) dissected from the arches and flat-mounted. In wild types, dorsal and ventral elements in the first arch (pq, palatoquadrate; M, Meckel’s) and second arch (hs, hyosymplectic; ch, ceratohyal) are separated by joints (arrows). In mutants, ventral cartilages are reduced and fused to dorsal cartilages at what we interpret to be the sites of the missing joints (arrows). Scale bars: 100 μm. PHENOTYPE:
|
|
Exogenous et-1 rescues ventral cartilage formation in suc mutants. (A-D) Ventral/oblique views of Alcian-stained larvae. In all panels, the black line marks the midline, the arrowhead marks the mouth, and white asterisks mark small ectopic elements (see Table 1). Dorsal and ventral cartilages are labeled as in Fig. 1 and joints are marked with arrows. (A) Uninjected wild type. Cartilages and joints are clearly identifiable (compare to Fig. 1C and D). (B) Uninjected suc mutant. Ventral cartilages are reduced, joints are absent, and the mouth is displaced ventrally. (C) pCS2-ET1 DNA-injected (20 pg) PCRgenotyped suc mutant (rescued left side). Ventral cartilages are present unilaterally, the mouth is no longer ventrally displaced, and the second arch joint has been partially rescued. (D) Unrescued right side of animal in C. This image has been vertically flipped for comparison with A-C. Ventral cartilages on the unrescued side are severely reduced while a ceratohyal (white arrow) is present on the contralateral rescued side. (E,F) Tracings of the cartilages in C and D, respectively, labeled as in A-D. (G,H) Dissected out and flat-mounted cartilages (labeled as in Fig. 1) from the rescued side of suc/et-1 mutants injected with 20 pg of pCS2-ET1 DNA (G) and human mature ET-1 protein (H) at 28 hours. Genotypes of all animals in C-H were confirmed by PCR-genotyping (see Materials and Methods). Scale bars, 50 μm. |
|
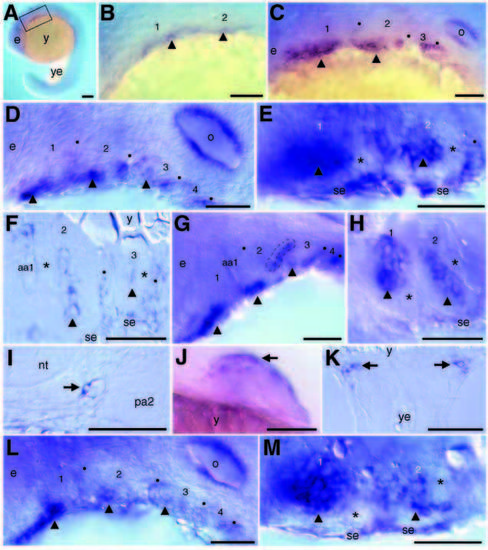
suc/et-1 expression in wild-type (A-K) and suc/et-1 mutant (L,M) embryos. In all panels, when visible, the arches are numbered, arch cores are marked with arrowheads, and black dots mark the pharyngeal pouches. (A) Lateral view of a whole-mount embryo at 18 hours. (B) Higher magnification of boxed area in A. Scattered ventral mesenchymal cells in the first two arches express suc/et-1 (arrowheads). (C) Lateral view at 24 hours. Strong expression is seen in a ventral mesenchymal cluster of cells in each of the first two arches. Expression is also seen in the second and third pharyngeal pouches. (D) Lateral view at 30 hours. Expression is seen in a mesenchymal core in each of the first three arches and in pharyngeal pouches 2-4. (E) Ventral views at 30 hours and (F) horizontal section at 32 hours through arches 2 and 3. The mesenchymal clusters of suc/et-1-expressing cells are surrounded by non-expressing cells (asterisks). Expression in the ventral surface ectoderm is continuous with expression in the second pharyngeal pouch. (G) Lateral view at 36 hours. Strong expression persists in a central core in the first three arches, and in pharyngeal pouches 2-4. A dashed line outlines the second pharyngeal pouch instead of marking it with a dot here to show the double cuboidal epithelial nature of the pouches, and the localization of suc/et-1 expression to ventral, posterior pouch epithelia. (H) A montage of two focal planes of the same whole-mount embryo (ventral view) at 36 hours. The mesenchymal cores continue to express suc/et-1 and have elongated in the mediolateral direction. (I) 32 hours; transverse section of endothelial cell (arrow) lining the dorsal aorta. (J) Ventral/lateral view of pectoral fin at 48 hours,. Anterior cells (arrow) express suc/et-1. (K) Horizontal section through yolk/hindyolk boundary showing bilateral clusters of suc/et-1 expressing cells (arrows) at 24 hours. (L-M) Lateral (L) and ventral (M) views of suc/et-1 expression in PCR-genotyped suc/et-1 mutants at 30 hours. Both epithelial domains are present, as are strongly expressing mesenchymal cores. Compare with D-E. aa1, aortic arch 1; e, eye; nt, neural tube; o, otic vesicle; pa2, pharyngeal arch 2; se, surface ectoderm; y, yolk ball; ye, yolk extension. Scale bars, 50 μm, except A,J (100 μm). EXPRESSION / LABELING:
|
|
dlx2, dHAND, and suc/et-1 expression in wild-type (A-E,G,I,K) and suc/et-1 mutants (F,H,J,L). In all panels, arches are numbered and when visible, the pharyngeal pouches are marked with black dots. (A) Two-color in situ hybridization with dlx2 in red and dHAND in dark blue in a wild-type embryo at 28 hours. At this stage, dHAND expression marks a ventral subset of dlx2-expressing mesenchymal cells in each of the first four arches. (B) dlx2 expression in horizontal section through arches 2 and 3 at 28 hours. Within each arch, dlx2-expressing cells surround a core of nonexpressing cells. (C) Ventral view of dHAND expression in wholemount embryo at 28 hours. Within each arch, dHAND-expressing cells also surround a central core of non-expressing cells. (D) Ventral view at 28 hours showing suc/et-1 expression, which is strikingly complementary to dHAND expression (compare with C). suc/et-1 expression is detected in a central core of mesenchymal cells in the first two arches, and in arch epithelia, both ventral surface ectoderm and the second pharyngeal pouch. (E-F), Lateral views, at 28 hours, of dlx2 expression in PCR-genotyped wild-type (E) and suc/et-1 mutant (F) whole-mount embryos. In wild types dlx2 is expressed broadly in most if not all postmigratory arch neural crest, and is not expressed in the pharyngeal pouches. suc/et-1 mutant embryos have a reduction of ventral dlx2 expression. (G-H) 28 hours; sagittal sections of dlx2 expression in PCR-genotyped wild-type (G) and suc/et-1 mutant (H) cut through the lateral aspect of arches 1 and 2. In suc/et-1 mutant embryos, ventral cells are present but do not express dlx2 (black arrows). In contrast, suc/et-1 mutant dorsal cells (white arrows) and cells lining the stomodeum express dlx2 at normal levels. (I-L) dHAND expression in whole-mount wild-type (I, K) and suc/et-1 mutant (J,L) embryos at 28 hours. I and J are lateral views, K and L are ventral views. dHAND expression in suc/et-1 mutants is severely reduced in the first two arches (arrows in J and L). dHAND is also normally expressed in the heart, the pectoral fin (data not shown), and a discrete cluster of cells at the arch one/two boundary (arrowhead in L); these domains are not affected in suc/et-1 mutants (K-L and data not shown). e, eye; h, heart; o, otic vesicle; se, surface ectoderm; st, stomodeum. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
suc/et-1 is required for ventral neural crest specification in the arch primordia. Lateral views of whole-mount embryos processed for single color (A-F) or two-color (G,H) in situ hybridization. In all panels, the arches are numbered, and pharyngeal pouches are marked with black dots. (A-B) 24 hours, msxE expression in wild type (A) and suc/et-1 mutant (B). Ventral mesenchymal cells in the first three arches express msxE in wild types, but do not in suc/et-1 mutants. (C-D) 30 hour, gsc expression in wild type (C) and suc/et-1 mutant (D). In wild types, gsc is expressed in separate patches of dorsal and ventral mesenchyme in the second arch. In suc/et-1 mutants, dorsal cells express gsc while ventral cells do not. (E-F) 24 hours, dlx3 expression in wild type (E) and suc/et-1 mutant (F). In wild types at this stage, dlx3 is expressed in ventral but not dorsal mesenchyme in arches one, two, and four. In suc/et-1 mutants, ventral cells fail to express dlx3. (G-H) 32 hours, dlx2 (red) and EphA3 (blue) expression in wild type (G) and suc/et-1 mutant (H). EphA3 expression is ventrally restricted similar to dHAND. In suc/et-1 mutants, EphA3 expression is severely reduced in the first four arches. Fig. 5 should be referred to for the dlx2 defect in suc/et-1 mutants. e, eye; o, otic vesicle. Scale bars: 50 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
suc/et-1 is required for later mesodermal and endodermal patterning in the pharyngeal arches. (A-D) myoD expression at 54 hours in wild types (A,C) and suc/et-1 mutants (B,D). In mutants, the mouth is displaced ventrally, and the ventral myoD-expressing premyogenic condensations in arches 1 and 2 are absent. (E-H) Larval muscles at 5 days detected with the 1025 antibody to muscle myosins in wild types (E,G) and suc/et-1 mutants (F,H). In mutant larvae, ventral muscles are severely mispatterned. The first arch ventral muscles, intermandibularis anterior and posterior (ima and imp), are missing in suc/et-1 mutants, other than a few occasional fibers spanning the midline. The ventral arch two muscles, interhyoideus and hyohyoideus (ih and hh), are dramatically shorter and do not meet in the midline as they do in wild-types (E-F). Dorsal muscles (am, lap, do, ao) appear normal in suc/et-1 mutants. (I-L) shh expression in wild types (I,K) and suc/et-1 mutants (J,L) at 54 hours. (I,J) Anterior views, focused deep to the mouth at the level of the first pharyngeal pouch, (K,L) lateral views. In mutants, expression in the first pharyngeal pouch does not extend as laterally as in wild types (arrows in I and J) and a midline diverticulum has not formed (arrows in K and L). am, adductor mandibulae; ao, adductor operculi; do, dilator operculi; h, heart; ih, interhyoideus; ima, intermandibularis anterior; imp, intermandibularis posterior; hh, hyohyoideus; lap, levator arcus palantini; p, pharynx; sh, sternohyoideus; tv1-4, branchial muscles transversus ventralis 1-4 (see Schilling and Kimmel, 1997 for wild-type muscle description). Scale bars: 50 μm. |
|
suc/et-1 functions nonautonomously in neural crest and mesoderm. (A) Schematic of neural crest transplants. Premigratory neural crest cells from biotin-labeled donors were tranplanted into unlabeled hosts at the 5 somite stage. (B) Schematic of mesodermal transplants. Cells from the head muscle domain of biotin-labeled gastrulas (Kimmel et al., 1990) were transplanted into the marginal region of unlabeled hosts. (C-H) Genetic mosaic animals at 24 hours (C-F) and 4 days (G,H), with the arches numbered. (C-F) Superimposed fluorescent and bright-field images to colocalize the biotin lineage tracer with dHAND expression in whole-mount embryos. (C,E) suc/et-1 mutant neural crest cells migrate into the dHAND-expressing region of arch one (C) and two (E). In both of these mosaic animals, suc/et-1 mutant ventral neural crest expressed dHAND (see Table 3). Both wild-type (not shown) and suc/et-1 mutant mesoderm (D) fills the non-dHAND-expressing central core in a wild-type host. Initially, neural crest cells lie lateral to mesoderm (F). (G) Mosaic larva in which suc/et-1 mutant neural crest cells, labeled in brown, contributed to a normal ventral cartilage (the ceratohyal) in an unlabeled wild-type host. (H) Mosaic larva in which labeled suc/et-1 mutant mesodermal cells have contributed to a normal ventral muscle (interhyoideus) in a wild-type host. bh, basihyal; ch, ceratohyal; ih, interhyoideus. |

Unillustrated author statements EXPRESSION / LABELING:
|