- Title
-
The deltaA gene of zebrafish mediates lateral inhibition of hair cells in the inner ear and is regulated by pax2.1
- Authors
- Riley, B.B., Chiang, M.Y., Farmer, L., and Heck, R.
- Source
- Full text @ Development
|
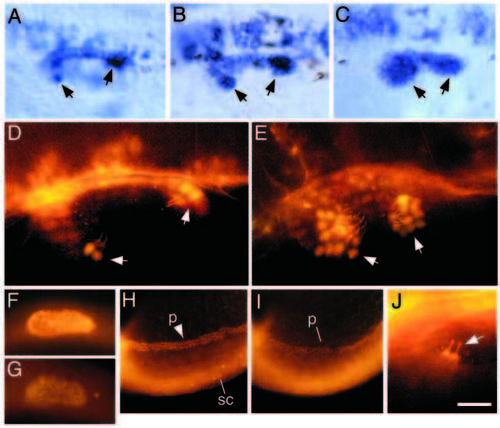
Expression of pax2.1 in the inner ear of wildtype and dlAdx2/dx2 embryos. (A-C) Dorsolateral view of the otic vesicle at 24 h showing accumulation of pax2.1 transcripts in a wild-type embryo (A), a moderately affected dlAdx2/dx2 mutant (B) and a severely affected dlAdx2/dx2 mutant (C). Cells lining the medial wall of the otic vesicle are heavily labeled, including small groups of cells in the developing sensory patches (arrows). In the dlAdx2/dx2 mutants, increased numbers of labeled cells are evident in the sensory epithelia. (D,E) Dorsolateral view of the otic vesicle at 24 h showing immunofluorescent staining with two antibodies: one directed against acetylated tubulin and the other generated against mouse Pax2. Within the otic vesicle, anti-acetylated tubulin specifically labels the apical surfaces and kinocilia of hair cells (Haddon and Lewis, 1996; Riley et al., 1997). In both wild-type (D) and dlAdx2/dx2 (E) embryos, hair cell nuclei show strong anti-Pax2 staining (arrows), and hair cells are overproduced in the dlAdx2/dx2 mutant. (F,G) Lateral view of the nascent otic vesicle at 18.5 h showing immunofluorescent staining with anti-Pax2 antibody in a wild-type embryo (F) and a noitb21/tb21 (pax2.1) mutant (G). (H,I) Lateral view of the posterior trunk and tail region showing immunofluorescent staining with anti-Pax2. Nuclei are labeled in the developing pronephros (p) and in a subset of neurons in the spinal cord (sc). (J) Dorsolateral view of anterior hair cells in a noitb21/tb21 mutant at 24 h labeled with antibodies directed against acetylated tubulin and Pax2. Hair cell apices and kinocilia are strongly labeled with anti-acetylated tubulin (arrow), but nuclear Pax2 staining is no longer detectable. Because noitb21 reduces expression of pax2.1 (Brand et al., 1996) but not pax2.2 (Pfeffer et al., 1998), these data suggest that the Pax2 antibody used here preferentially recognizes pax2.1. (A-G,J) Anterior is to the left and dorsal is upward. (H,I) Anterior is to the right and dorsal is downward. Scale bar, 10 μm (J), 20 μm (D,E), 35 μm (A-C), 60 μm (F,G), and 115 μm (H,I). |
|
Differentiation of hair cells in wild-type embryos. (A) Dorsolateral view of the otic vesicle at 30 h showing anti-Pax2 staining. The nuclei of presumptive hair cells stain intensely (arrows) whereas surrounding cells show lower staining levels. (B) Parasagittal section of a 30 h embryo stained in whole mount with anti-Pax2 and anti-acetylated tubulin antibodies. Hair cells in the utricular macula are marked by their strong nuclear Pax2 staining (arrow), as well as their stained kinocilia and associated otolith (o). Support cells show no detectable staining. (C) Dorsal view of the otic vesicle at 48 h showing anti-Pax2 staining in the utricular macula. The nuclei of hair cells stain intensely (arrow) and presumptive nascent hair cells (n) surrounding the macula show lower staining levels. (D) Longitudinal section of a 60 h embryo stained in whole mount with anti-Pax2 and anti-acetylated tubulin antibodies. In this slightly oblique dorsal view of the saccular macula, the kinocilia are not visible, but hair cells are clearly marked by their nuclear anti-Pax2 staining (arrow) and the close proximity of the otolith (o). Support cells are not labeled. (E) Lateral view of the otic vesicle at 60 h showing anti-Pax2 staining in the cristae (c). (F) Lateral view of posterior crista in a 60 h embryo stained with anti-Pax2 (red) and anti-acetylated tubulin (green). Hair cells (arrow) show nuclear Pax2 staining and ciliary acetylated tubulin staining. Support cells are not labeled. In all panels, anterior is to the right. (A,B,E,F) Dorsal is up; (C) medial is upward, and (D) medial is downward. Scale bar, 15 μm (F), 20 μm (A-D), or 65 μm (E). |
|
Overproduction of hair cells in dlAdx2/dx2 mutants. (A) Dorsolateral view of the otic vesicle of a 30 h dlAdx2/dx2 embryo stained with anti-Pax2 antibody. Hair cells stain intensely (arrows) and are produced in much greater numbers than normal. (B) Dorsolateral view of the otic vesicle of a 30 h dlAdx2/T(msxB)b220 trans-heterozygote stained with anti-Pax2 antibody. Stained hair cells (arrows) are produced in excess, but to a lesser degree than in dlAdx2/dx2 homozygotes. (C) Parasagittal section of a dlAdx2/dx2 embryo stained in whole mount with anti-Pax2 and anti-acetylated tubulin antibodies. Large numbers of hair cells are labeled (arrow), but few support cells are evident. An otolith (o) is attached to the hair cell ciliary bundles. (D). Lateral view of the otic vesicle of a live dlAdx2/dx2 embryo as seen under DIC optics. Numerous hair cells are evident (arrow) and a large malformed otolith (o) is distributed across the tips of the hair cell ciliary bundles. (E) Lateral view of the posterior crista of a 60 h dlAdx2/dx2 embryo stained in with anti-Pax2 (red) and anti-acetylated tubulin (green) antibodies. Hair cells show nuclear Pax2 staining and are produced in greater than normal numbers. Anterior is to the right and dorsal is to the top. Scale bar, 15 μm (E), 20 μm (A-C), or 25 μm (D). |
|
Genetic interactions between noitb21, dlAdx2 and mibta52b. (A) Dorsolateral view of the otic vesicle of a 30 h noitb21/tb21 embryo stained with anti-Pax2 and anti-acetylated tubulin antibodies. Nuclear staining is not detectable, but the apical surfaces and kinocilia of hair cells are strongly labeled (arrows). Twice the normal number of hair cells are evident. (B) Parasagittal section of a noitb21/tb211 mutant stained in whole mount with anti-Pax2 and antiacetylated tubulin antibodies. Nuclear pax2 staining is not detectable, but hair cells are clearly marked by acetylated tubulin staining (arrow) and the close association with the otolith (o). (C) Otic vesicle of a live noitb21/tb21 mutant viewed under DIC optics at 19 h. The kinocilia (k) of two tether cells, and attached otolith precursors, are seen at the anterior end of the otic vesicle. (D) Otic vesicle of a live dlAdx2/dx2 mutant viewed under DIC optics at 19 h. The kinocilia (k) of supernumerary tether cells are evident. (E) Dorsolateral view of the otic vesicle of a 30 h dlAdx2/dx2; noitb21/tb21 double mutant stained with anti-Pax2 and anti-acetylated tubulin antibodies. Hair cells, detected by staining of their kinocilia and apical surfaces (arrows), are produced in numbers that are comparable to noitb21/tb21 single mutants. (F) Dorsolateral view of the otic vesicle of a 30 h mibta52b.ta52b; noitb21/tb21 double mutant stained with anti-Pax2 and anti-acetylated tubulin antibodies. Hair cells (arrows) are produced in much greater numbers than in noitb21/tb21 single mutants. In all panels, anterior is to the right and dorsal is up. Scale bar, 10 μm (C,D), 15 μm (B), or 25 μm (A,E,F). PHENOTYPE:
|
|
Time course of hair cell formation in utricular maculae. Tether cells were visualized in live embryos at 18.5 and 21 h. At later stages of development, hair cells were visualized by fixing and staining embryos with anti-Pax2 and/or anti-acetylated tubulin antibodies. Utricular (anterior) maculae are easily visualized in live specimens and show faster rates of growth than saccular maculae in all genetic backgrounds. Each time point shows the mean and standard deviation of data pooled from three clutches of 10 or more embryos each. Symbols: (●) wild-type, (○) noitb21/tb21, (■) dlAdx2/dx2 and (□) dlAdx2/dx2; noitb21/tb21 embryos. PHENOTYPE:
|
|
Expression of dlA and dlD in tether cells in the anlage of the maculae. Dorsal views of nascent otic vesicles at 19 h showing expression of dlA (A-C) or dlD (D-F) in tether cells (arrows). Wild-type embryos (A,D) and noitb21/tb21 mutants (B, E) show expression in pairs of tether cells in the anteromedial and posteromedial regions of the otic vesicle. In dlAdx2/dx2 mutants (C,F), delta-expressing tether cells are produced in excess. Scale bar, 20 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
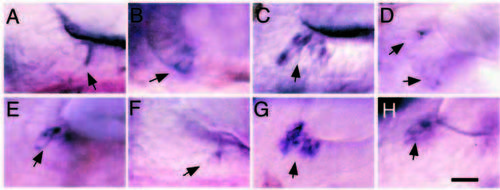
Expression of dlA and dlD in hair cells in anterior maculae. Lateral views of utricular maculae at 30 h showing expression of dlA (A-D) or dlD (E-H) in nascent hair cells (arrows). Wild-type embryos (A,E) usually show only one or two labeled cells at this time. noitb21/tb21 mutants (B,F) show a normal number of stained cells, but expression levels are usually lower than normal. dlAdx2/dx2 mutants (C,G) show supernumerary nascent hair cells with intense labeling, whereas dlAdx2/dx2; noitb21/tb21 double mutants (D,H) show near normal numbers of nascent hair cells with reduced labeling. In all panels, anterior is to the right and dorsal is up. Scale bar, 15 μm. |

Unillustrated author statements PHENOTYPE:
|