Fig. S1
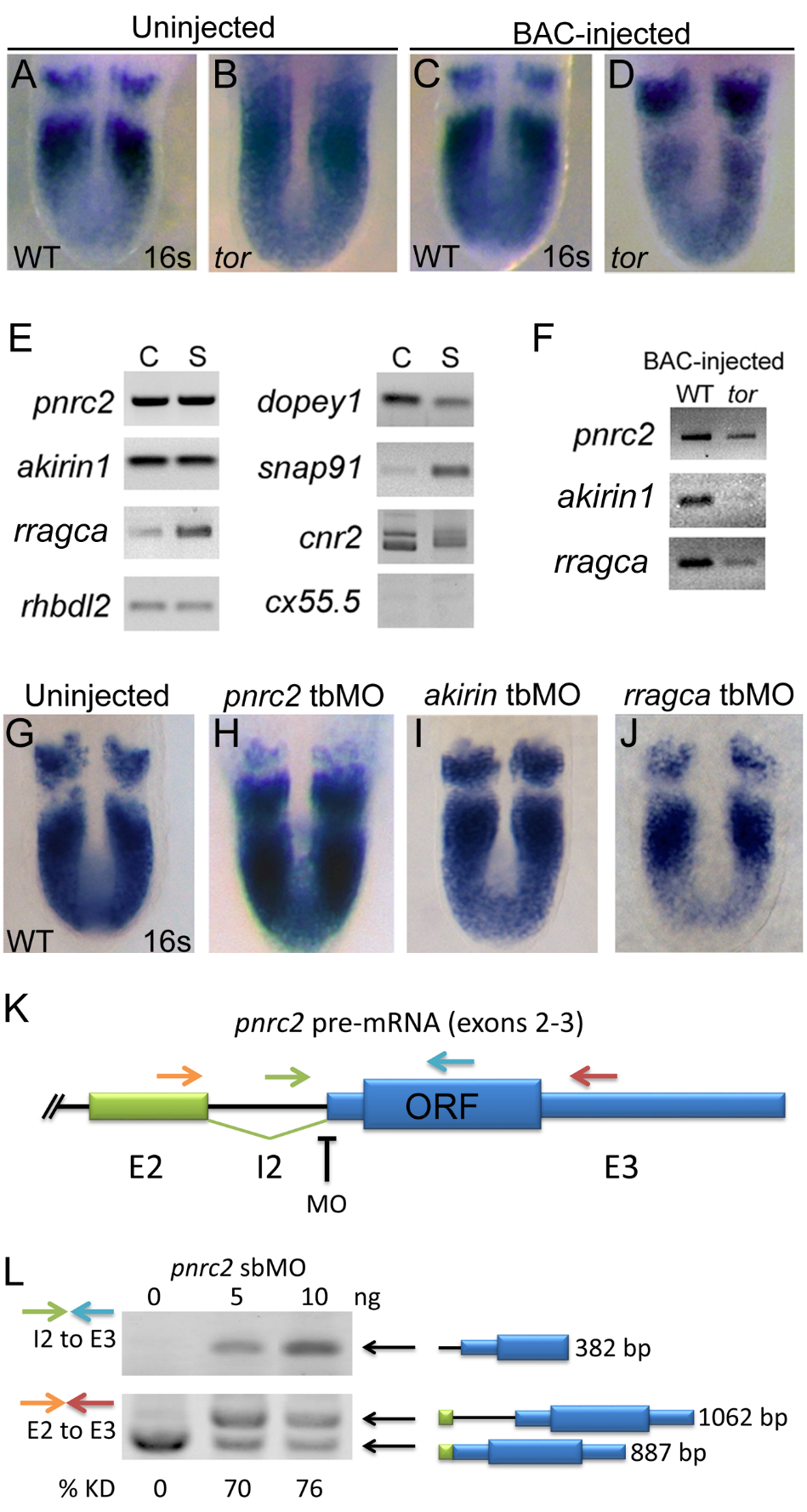
pnrc2 is the best candidate within the tortuga interval for the gene regulating cyclic transcript decay. Injection of 30 pg BAC AL844887 does not affect her1 expression in wild-type sibling embryos (n=27/27) (A versus C), but does significantly reduce accumulation of her1 transcripts in tortugab644 mutants (n=10/10) (B versus D; Table S1). BAC-injected tor mutants are indistinguishable from wild-type siblings and are identified by PCR-based genotyping of individual embryos after her1 in situ hybridization (data not shown). (E) RT-PCR expression analysis of genes on BAC AL844887 reveals that seven are expressed during cleavage (C) and segmentation stages (S), with particularly robust detection of pnrc2 and akirin1. In addition to RefSeq-annotated genes on BAC AL844887, snap91 and dopey1, both of which lack RefSeq annotation, are detectably expressed during cleavage and segmentation stages (E). (F) RT-PCR expression analysis of genes on BAC AL844887 in tortuga mutants indicates that pnrc2, rragca, and to a lesser extent akirin1 are expressed from injected BAC DNA (see Supplemental Methods). dopey1, snap91, cnr2, and rhbdl2 are not expressed from the BAC (data not shown). (G-I) Injection of translation-blocking morpholinos (tbMOs) targeting akirin1 and rragca into 1-cell stage wild-type embryos has no effect on the striped her1 expression pattern observed in WT embryos (G vs I, J), but injection of a pnrc2 tbMO has a dramatic effect (G vs H). A range of doses (1.5 ng-6 ng) was tested for each MO (n>50 per dose). (J) Schematic depicts primer and splice-blocking MO (sbMO) target location on the pnrc2 transcript. (K) RT-PCR splicing analysis was performed to detect both spliced and unspliced pnrc2 transcript in uninjected wild-type and pnrc2 morphant embryos (see Supplemental Methods). One primer combination was used to specifically amplify unspliced product (green and blue arrows in K) and exon-specific primers (orange and red arrows in K) were used to amplify both spliced and unspliced product for semi-quantitative splicing analysis (Gallagher et al, 2011). Injection of pnrc2 sbMO reduces normal pnrc2 splicing by >70% knockdown in a dose-dependent manner (n=20 per dose) (L). Although intron retention alone does not disrupt pnrc2 coding potential as this lies entirely in exon 3 (K), aberrant transcripts induced by pnrc2 sbMO-injection coincide with elevated her1 mRNA levels (Fig 1D) suggesting that pnrc2 sbMOs are effective. Injection of either pnrc2 splice-blocking (sbMO) or a pnrc2 tbMO have similar, dose-dependent effects on her1 expression when injected at doses ranging from 1.5-6 ng (n>50 per dose) (data not shown). All PCR products were sequenced using primers listed in Table S3. ORF=pnrc2 open reading frame; KD=knockdown.
Reprinted from Developmental Biology, 429(1), Gallagher, T.L., Tietz, K.T., Morrow, Z.T., McCammon, J.M., Goldrich, M.L., Derr, N.L., Amacher, S.L., Pnrc2 regulates 3'UTR-mediated decay of segmentation clock-associated transcripts during zebrafish segmentation, 225-239, Copyright (2017) with permission from Elsevier. Full text @ Dev. Biol.